Conformations are three-dimensional arrangements of atoms that arise due to the free rotation of a carbon-carbon(C-C) single bond. Cyclohexane exists in puckered or non planner rings. This ring puckering helps to avoid ring strain, angle strain, and eclipsing strain. The twisting of a carbon-carbon single bond results in the different conformation of cyclohexane i.e chair conformation, boat conformation, twist boat conformation, and half chair conformation.
Learning objective
After learning this you will be able to understand the different conformations of cyclohexane.
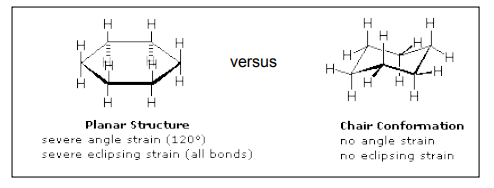
Conformations are 3-dimensional structures that arise due to free rotation of single bonds. Cyclohexane is a six-membered ring molecule. In planner six member hexagonal shape it has bond angles of 1200 and so it must have angle strain because all angle are greater than 109.50. To avoid unfavourable interactions cyclohexane and its derivatives exist in nonplanner form. This ring puckering helps to overcome angle strain, eclipsing strain, and ring strain.
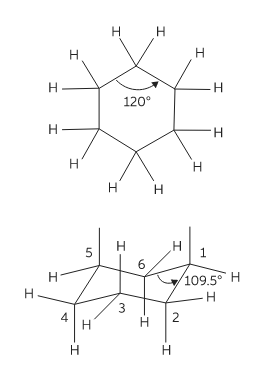
Conformations of cyclohexane
There are four main conformations of cyclohexane
- Chair conformation
- Boat conformation
- Half chair conformation
- Twist chair conformation
Chair conformation (most stable conformation)
The name chair conformation arises because the structure resembles a chair. Four of the carbon atoms are coplanar and form the seat of the chair whereas the other two carbon atoms are above and below this plane respectively. The carbon atom above the plane forms the headrest of the chair whereas the carbon atom below the plane forms the footstool of the chair.
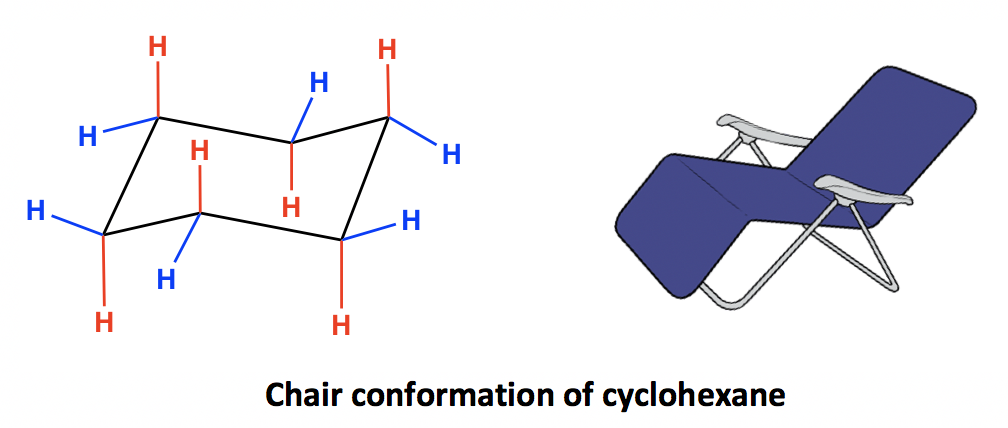
Structure of chair conformation:
In the chair form, all C-C-C bond angles are approximately 1090 so there is no angle strain. Similarly, all adjacent CH2 groups are staggered with respect to one another. So there is no torsional strain. In addition, there are close interactions, so there is no ring strain.
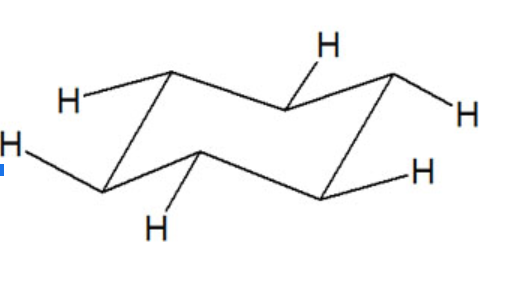
All six carbon atoms in the chair conformation of cyclohexane are equivalent. but the hydrogen atoms are not equivalent.
There present two different types of hydrogen.
- Axial hydrogen
- Equatorial hydrogen
Axial Hydrogen
There are six hydrogens that are perpendicular to the plane of the ring are called axial hydrogen. Three hydrogens are perpendicular in the upward direction.
Three hydrogens are perpendicular in the downward direction.
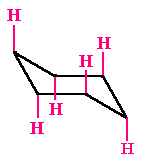
Equatorial Hydrogen
There are six hydrogens that are located at ±300 to the plane of the ring. They project out sideways along the plane of the ring
 .
.
Geometrical position of Hydrogens of cyclohexane
The axial hydrogens perpendicular in the upward direction are cis to each other. Similarly, the axial hydrogens perpendicular in the downward direction are also cis to each other whereas all upward hydrogens are trans to all downward hydrogens. The same is in the case of equatorial hydrogens.
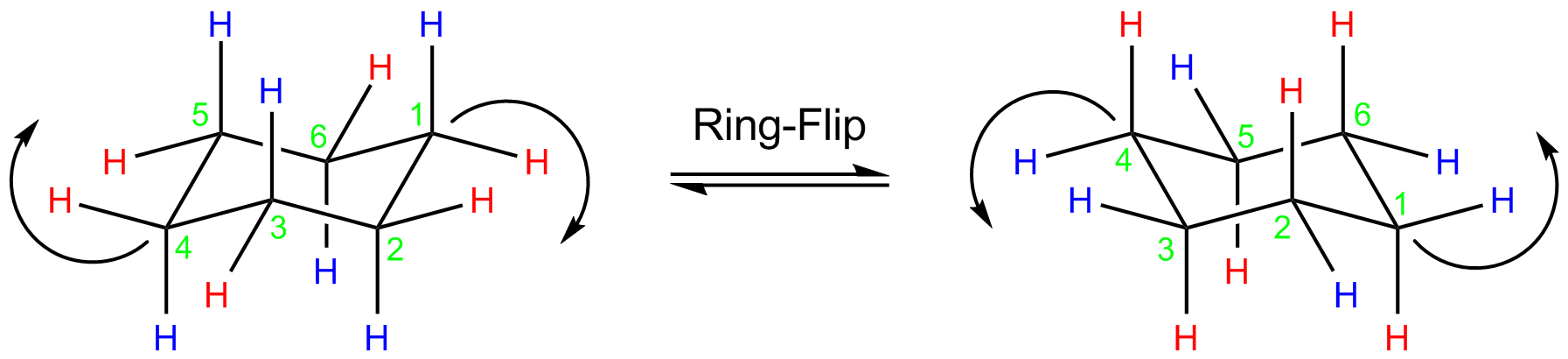
Ring flipping of chair form
Although, there present two types of hydrogen atoms but the 1H NMR spectrum of cyclohexane shows only one peak at room temperature. This is because there exist dynamic equilibrium between two energetically degenerate chair conformations. So all axial are converted into equatorial and equatorial into axial at room temperature.
But at low temperature rate of interconversion reduced and is possible to see two peaks in 1H NMR coressponding to two types of hydrogens.
Boat conformation
When the footstool carbon of the chair form of cyclohexane is moved up above the plane of the seat of the chair then we have new conformation named as boat conformation. Boat form is free from angle strain. But in boat conformation hydrogen are in eclipsed state. This result in tortional strain in molecule.
Hydrogen of Carbon-1 and Carbon -4 have vander waals repulsions. This is known as flag pole interaction. This non bonded interaction between these hydrogens give rise to steric strain. These two parameter make boat form less stable as compared to chair form.
Twist boat conformation
Boat conformation is very flexible. It can undergo slight twist about bond. If one of the C-C bond forming base of boat is twisted about its mid point we can have a different conformation named as twist boat conformation. In twist boat there is less eclipsing of CH2 groups. Similarly there is less flag pole repulsion because hydrogens are far apart from each other. So this make twist boat confirmation little more stable then boat conformation.
Half chair conformation (least stable conformation)
This is least stable conformation of cyclohexane. This is formed by bringing footstool of chair into the plane of seat of chair. In this way 5 carbon atoms comes in sameplane. So it has maximum strain. Its bond are fully eclipsed. In this conformation C-C bond angles of plane are at 1200 having angle strain. Thats why it is least stable conformation of cyclohexane.
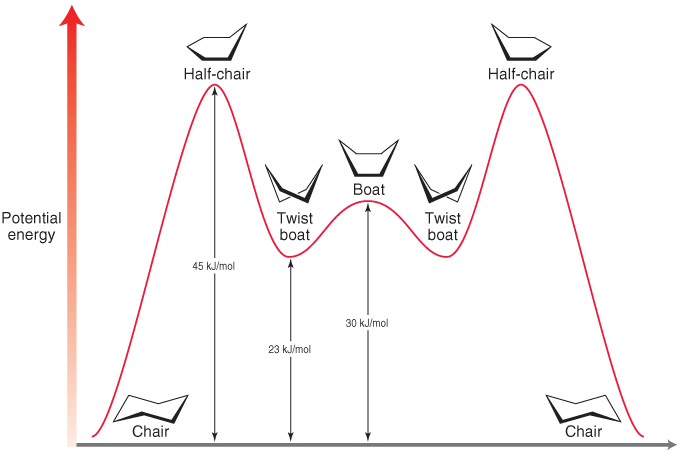
Energy diagram
Concepts Berg
Which is the most stable conformation of cyclohexane?
Chair conformation of cyclohexane is most stable conformation of cyclohexane.
Which conformation of cyclohexane is the least stable?
Half chair conformation is the least stable conformation of cyclohexane.
Which conformation of cyclohexane is chiral?
The twist conformation of cyclohexane is chiral in nature because it has no plane of symmetry.
What is Conformation of Cyclohexane?
Different three-dimensional arrangements of cyclohexane formed due to free rotation across a single bond without breaking the bond are called as conformation of cyclohexane.
Which conformation is more stable axial or equatorial?
In general equatorial are more stable than axial positions because of less internal interactions. Because in equatorial position there is no steric hindrance and also substituent prefers equatorial to avoid 1-3 diaxial interactions.
What makes the half chair conformation of cyclohexane so unstable?
In half chair conformation 5 carbon atoms are in same plane. They have steric strain. They are fully eclipsed. Similarly bond angles of planner Carbons are 1200. So they have angle strain to. This makes half chair least stable.
What are the different conformations of cyclohexane?
There are four different conformation of cyclohexane:
- Chair conformation (most stable)
- Boat conformation
- Twist boat conformation
- Half chair conformation (least stable)
Which one is the most stable and why?
Chair form is most stable conformation because
All CH2 groups are staggered so there is no steric strain
All bond angles are 109.50 so there is no angle strain
Similarly, it is free from ring strain. That’s why it is the most stable conformation of cyclohexane.
What are axial hydrogens?
Axial Hydrogen
There are six hydrogens that are perpendicular to the plane of the ring are called axial hydrogen. Three hydrogens are perpendicular in the upward direction.
Three hydrogens are perpendicular in the downward direction.
What are equatorial hydogrens?
Equatorial Hydrogen
There are six hydrogens that are located at ±300 to the plane of the ring. They project out sideways along the plane of the ring
Why we have one peak in 1H NMR of chair form?
Although, there present two types of hydrogen atoms but the 1H NMR spectrum of cyclohexane shows only one peak at room temperature. This is because there exist dynamic equilibrium between two energetically degenerate chair conformations. So all axial are converted into equatorial and equatorial into axial at room temperature.
Which cyclohexane conformer is most energetic?
Half chair is the most energetic conformation of cyclohexane.
Why chair conformation of cyclohexane is more stable than its boat conformation?
Chair conformation is more stable than boat conformation because chair form is free from all types of strain while boat conformation has torsional strain and flag pole interactions that decrease the stability of boat conformation.
What is the order of stability of the conformers of cyclohexane?
Order of stability
- Chair conformation (most stable)
- Twist boat conformation
- Boat conformation
- Half chair conformation (least stable)
- What are the physical properties of cyclohexane?
Physical properties of cyclohexane:
- Cyclohexane is non polar, non planer, organic compound
- It is a colorless liquid with a sweet smell.
- Cyclohexane is slightly soluble in water.
- Cyclohexane is fully soluble in organic solvents.
- Melting point of cyclohexane is 80.740C
- Freezing point of cyclohexane is 6.540C
Why does camphor have boat conformation?
The camphor has two dissimilar chiral centers; only one pair of enantiomers is possible hence only cis-fusion of gem-di-methyl methylene to cyclohexane ring is possible making boat conformation for camphor the plausibly ONLY way to create a camphor molecule. Hence, it is like that in spite of being less stable because it has no other way to exist. (copied from quora)
