Complexometric Titrations (Chelometric / Chelatometric Titrations) technique involves titrating metal ions with a complexing agent to form complexes that are slightly ionized in solution.
Metal ions, especially transition metals act as Lewis acid because they accept electrons from Lewis Base. When metal ion combines with Lewis Base, a simple ion is transformed into a complex ion and the equivalence point is determined by using metal indicators.
- Lewis base might be an anion or neutral molecule, normally called Ligand which has the ability to share the electronic pair.
- Lewis Acid is a metal ion, called a central atom.
Metal(M+) + Chelating Agent (Y-)——-> Metal Coordination Complex(MY)
Coordination Bond: The bond formed between a metal ion (acceptor) and a Ligand (Donor).The number of bonds that a metal tends to form with Ligand is called Coordination Number. The bond is represented by – – – – >.
Example:
NH3
|
NH3 — Cu — NH3
|
NH3
Chelation:
Chelate is complex formed between the ligand containing two or more donor groups and metal to form ring structure. (heterocyclic rings or chelate rings). And the molecule that have two or more groups that form a ring structure with metal atom is called Chelating Agent.
There is no fundamental difference between co-ordination compound and a chelate compound except that in a chelate compound, ring influence the stability of compound. Thus, a chelate can be described as a heterocyclic ring structure in which a metal atom is a member of ring. The stability of a chelate is usually much
greater than that of corresponding unidentate metal complex.
- Chelates are usually soluble in Organic solvents
- Solubility of metal chelates in water depends upon the presence of hydrophilic groups presence. If they are present,the complex will be soluble easily. If hydrophilic groups are not present, both metal chelates and chelating agent will pe sparingly soluble.
Example :
Pencillamine Structure
Classification of Ligands
- Unidentate
The ligands which are bound to attach with metal ion with only one side. NH3, Cl,H20, CN-, etc
NH3–> Ag ← NH3
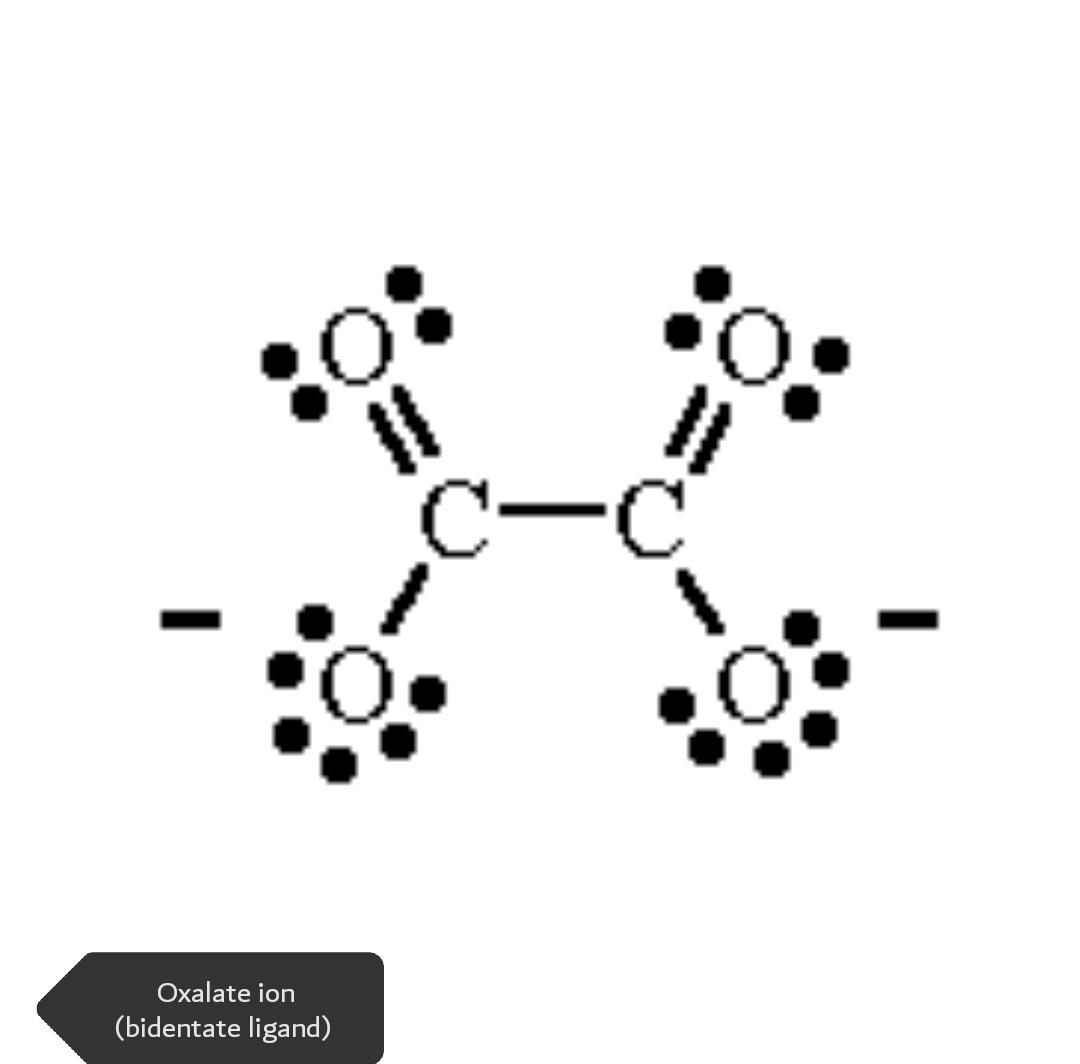
- Bidentate and Multidentate
The ligands that are capable of binding with metal ion with two sites are called bidentate and that with multiple sites to attach are called multidentate ligands. Bidentate
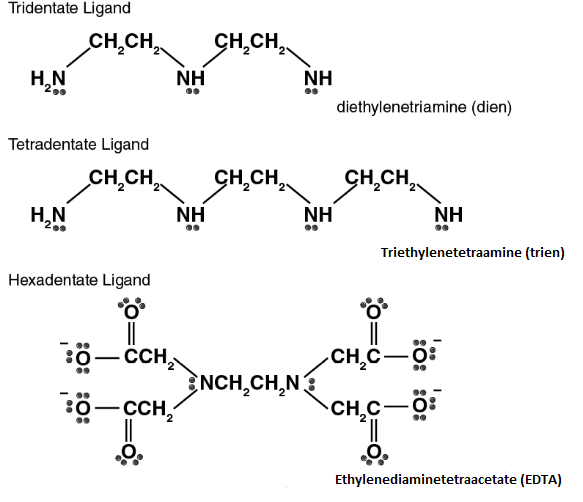
ligands (2 donar atoms), tridentate ligands (3 donar atoms), quadridentate ligands, etc.
Examples:
Complexation Titrations and EDTA
Disodium salt of EDTA (Ethylene diamine tetra acetic acid) is a hexadentate chelating agent with four acetate ion and two nitrogen donor atoms.
EDTA is highly preferred because of
- Water soluble chelating agent
- Very stable sequestering agent
- Low price
- Forms stable five membered rings
- Separation of multiple metal ions are possible
General equation for Complexometric Titrations is

Factors influencing the Complexes
- Formation and dissociation of complexes is affected by pH.
- In acidic medium, if [H+] is higher, ionization of EDTA will be lower, stability of metal-EDTA complex lowers which shifts the reaction backwards.
- In strong alkali solution, metals are precipitated as hydroxide
- In a slightly alkaline solution, stability of the complex is higher which results in a forward reaction.
- Central metal ion also influence the stability
- If the size of a metal ion is smaller, a more stable complex will form.
- Higher the electronegativity, stable will be the complex
- Metals having incomplete outer shell have higher tendency to form stable complex
- Large, bulky ligands form less stable complexes than smaller ones due to steric effect.
- When a complex is formed, it always have specific absorption spectrum which generally gives advantage in colorimetric analysis.
Methods of end point detection
- Indicators
Indicators are dyes which react with metals to form dye-metal complexes,which are different in color from the dye itself. They are less stable than the metal-chelate complex. During complexometric titration the color of the solution remains the same as that of that dye-metal complex. At equivalence point, the dye-metal complex decomposes to produce free dye, which gives a clear color change.
Many of these indicators also have the typical properties of acid-base indicators and the colour changes are the result of the displacement of the H+by a metal ion.
Metal indicators must have following properties :
- It must act as ligand to form complex
- It should form complexe which is less stable than metal complex
- Dye-metal complex must b stable during the complete reaction
- There must be a complete color difference in color of indicator and dye-metal complex.
- Reaction between metal and indicator must be reversible
- It must change its color according to the pH of the solution.
Example
Erichrome Black T
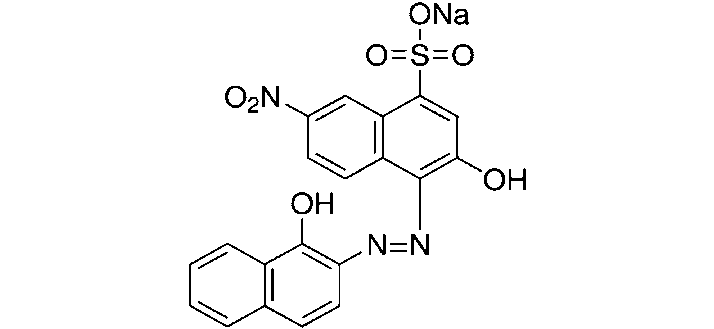
It is an azo-dye. Its contains two replaceable phenolic hydrogens. It is usually used for the determination of hardness of water.
Before Titration
Mg2+ + In- —> MgIn
(colorless) (blue) (red)
During Titration
Mg2+ + EDTA —> Mg-EDTA
(free Mg-ions) (solution is red due to MgIn complex)
At the End point
MgIn + EDTA —> Mg-EDTA + In-
(red) (colorless) (blue)
- Instrumental Methods
- Potentiometric Method
In this method change in redox potential of the solution being titrated is measured. Generally Platinum electrodes are used with standard reference electrodes. Platinum electrode measures the redox potential with metal-EDTA complex. Generally this equation is used:
E= Eo + log [Ox] /[Red]
where, E = the potential of the electrode
Eo = the standard electrode potential
[Ox] = conc of the ions in the oxidized state [Red] = conc of the ions in the reduced state
Let us understand it briefly. Here a complexing agent is being added to metal ions solutions. When a complexing agent shares its electrons to metal, oxidation occurs. At every point, there is a change in redox potential which is further measured by electrodes.
- Another electrode used is mercury electrode.
It measures when a replacement reaction occurs between a mercury ion and metal ion present in the Hg-EDTA complex.
- Photometric Titrations
The change in absorption spectrum when a metal ion of a complexing agent is converted to the metal complex.
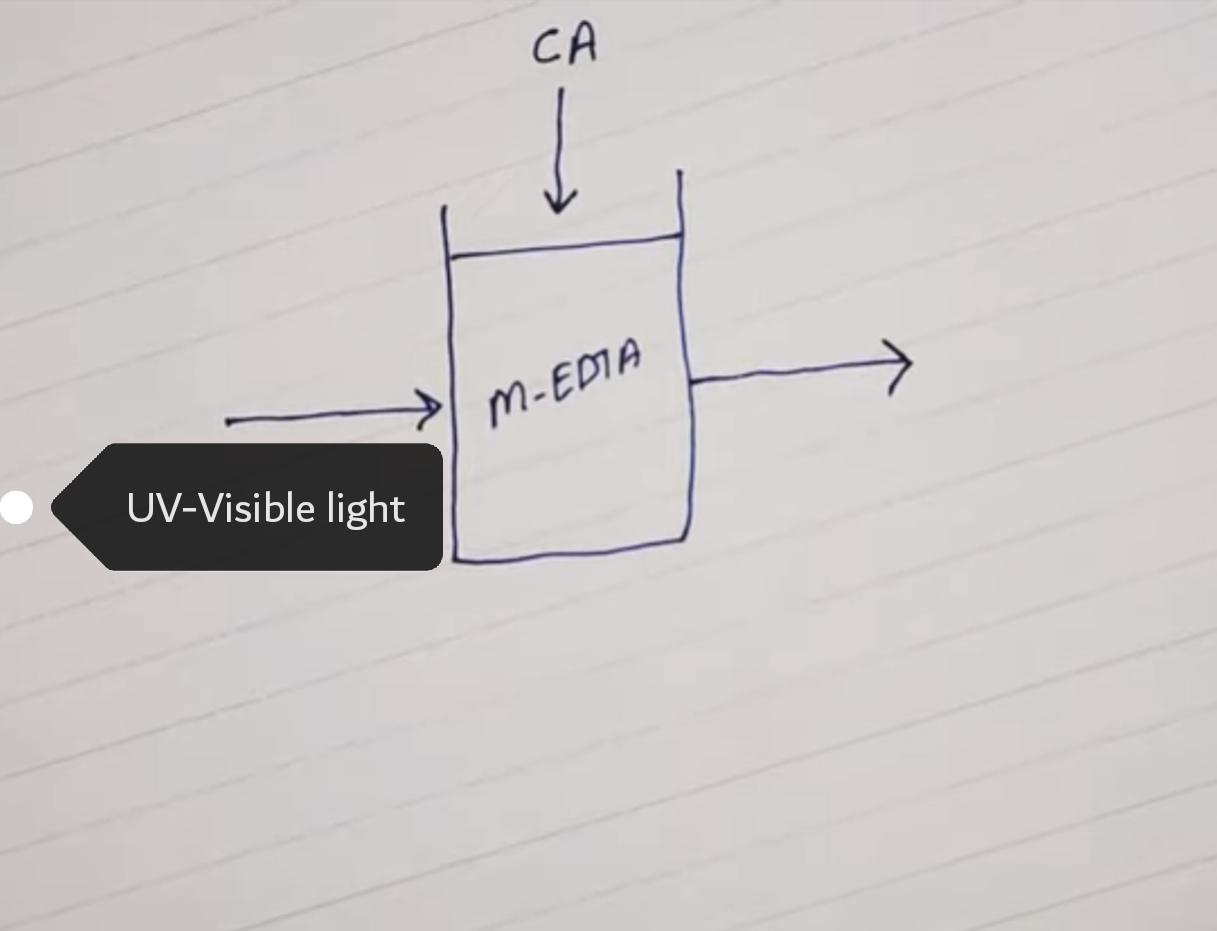
When light passes, there is a decrease in intensity due to the presence of a complex. As further reactions proceed, more decreases can be seen. Change in absorbance helps to measure the end point.
Indicator giving a colour change in the visible region is generally employed. Metals like Fe(III), Cu(II) etc generally form colored complexes upon which colorimetric analysis is carried out. But some metals form colorless complexes, so these can be estimated in UV-regions.
- Amperometric Titrations
Metals ions give diffusion current in its pure solution form. When EDTA is added and complex forms step by step, metal ion concentration decrease, so as the diffusion current. In this way Amperometric comolexometric titrations are carried out.
Types of Complexometric Titrations
There are four types of complexometric titrations techniques
- Direct Titrations
This is the mostly used and convenient method used in colorimetric analysis. Direct titration ia performed by titrating metal with ion containing buffer and few drops of indicator with standard Disodium salt of EDTA. End point is detected by change in color of the solution.
Similar titration is performed by taking only buffer and indicator (blank) to verify the impurities.
Some of the requirements for direct EDTA titrations are
- The compound to be determined must be water soluble
- Reaction between EDTA and metal must be rapid. If reaction is slow, it must be catalyzed.
- Metal ion should not be ppt. out at the pH of titration.
Example :
Direct determination of Zn2+ with EDTA
- The complex of Zn2+ with EDTA is more stable than its complex with EBT ind.
Zn2+ + H Ind2- —> Zn Ind- + H+
Zn Ind- + H2Y2- —> ZnY2- + H Ind.2- + H+
(wine Red) (Blue)
- Back Titration
In this method, excess of a standard EDTA solution is added to the metal solution, which is to be analyzed, and the excess is back titrated with a standard solution of a second metal ion.
In this method, solution is heated and cooled with excess EDTA solution to promote the formation of a complex between metal and EDTA. Here pH is adjusted by using buffer. Finally excess EDTA is titrated using Mg or Zn sulfates in presence of suitable indicators.
Example : Insoluble salts of calcium oxalates, Aluminum, Mn etc. are titrated by using this method.
- Replacement Titrations
This titration is performed when direct and indirect titrations don’t give satisfactory results. It is done by substituting equal amounts of Mg or Zn from a less stable complexe.
Mn2+ + MgEDTA2- —> Mg2+ + Mn-EDTA2-
Example : Calcium, Mercury and Lead can be determined by this method.
- Alkalimetric Titrations
In this method, protons (H+) produced by the formation of Metal-EDTA complex are titrated in standard alkali in non-buffered solution using visual indicators.
Alternatively, mixture of iodine-iodate liberates iodine when EDTA solution is added to it. Liberated iodine is titrated against standard sodium thiosulphate solution using starch as indicator.
Applications of Complexometric Titrations
Determination of Hardness of water
Hardness in water is due to the presence of dissolved salts of calcium and magnesium. It is calculated as ppm of Ca++ and Mg++ present in water. The sample is titrated against standard EDTA solution with EBT as indicator.
In this method Disodium salt of EDTA has the general formula: Na2H2Y2.H2O, where Y is the tetravalent anion of EDTA. When Ca is titrated with H2Y2-
, a very stable complex is formed.
Ca2+ + H2Y2- < —> CaY2- + 2H+
Ca-EDTA complex is much stable than Mg-EDTA complex. At pH 12 EDTA forms complex with Ca2+ only. At pH 10 EDTA forms complex with both Ca2+ and Mg2+. For this ammonium buffer is used. Upon titration with EDTA, Ca2+ is chelated first and then Mg2+.
Determination of Ca using EDTA
Ca2+ forms complex with EDTA at pH 10 and pH 12. But at pH 10, if Mg2+ is present, it is also titrated. For specific Ca2+ content determination, 5mL of 1M NaOH is used and Mureoxide as indicator.
Determination of Mg using EDTA
Mg2+ forms complete with EDTA at PH 10. But the problem is that Ca2+ also forms complex at this pH. For content of Mg2+, Ca2+ content are measured and been subtracted.
Total – Ca2+ = Mg2+
Determination of Aluminum Salts
Sample of Al3+ is heated with known excess of st. EDTA at pH 7-8. The soln. is then adjusted to pH=10 using ammonia buffer.The residual EDTA is titrated against st. Zn2+ using EBT indicator.The colour changes from blue to wine red.
pH 7-8
Al3+ + H2Y2- —> AlY- + 2 H+
Boil
pH 10
Zn2+ + H2Y2- —> ZnY2- + 2 H+
Zn2+ + H Ind.2- —> Zn-Ind.- + H+
Blue wine Red
Determination of Fe3+ using CNS-
Thiocynate CNS- indicator is specifically used for Fe3+ determination. When sample of Fe3+ is treated with CNS-, a blood red complex is formed. Upon titration with st. EDTA, the end point is detected by decolorization f blood red color.
Concepts Berg
What is Complexometric Titration?
Complexometric titration are those in which a coloured complex is formed, usually by the use of a chelating agent, such as EDTA, the end point being marked by a sharp decrease in the concentration of free metal ions.
What are acid-base indicators?
A substance that indicates the degree of acidity or basicity of a solution through characteristic color changes.
What are different types of complexometric titration?
There are four different types of complexometric titration.
- Direct Titration
- Back Titration
- Replacement Titration
- Alkalimetric Titration
What are the different indicators used in the complexometric?
Some examples of complexometric indicators are:
- EBT
- Mureoxide
- Thiocyanate
- Sodium diphenylamine sulfonate
- Diphenylamine
What is the principle of EDTA?
EDTA is Ethylene diamine tetra acetic acid. It dissolves in water with great difficulty, but its disodium salt dissolve in water quickly & completely It is hexa dentate ligend. It binds the metal ions in water to give stable chelate complex. Hardness of water is the example of EDTA titrations.
What are the advantages of complexometric titration?
- This is a simple technique that does not require high quality skills and expertise.
- Fast and Accurate
- Major advantage of complexometric titrations is the determination of mixtures of different metal ions in solution.
- It can also be performed using instruments.
What are some sources of errors in titration?
There can be multiple errors in titrations. Some of them are :
- Incorrect standardization
- Impure Chemicals
- Contamination of instruments /glassware use
- Titrant decomposition
- Weighing Error
- Faulty Calibration
- Electrodes uncertainty
What are the pharmaceutical applications of complexometry?
- It is widely used in the pharmaceutical industry to determine the metal concentration in drugs.
- Titanium dioxide is used in many cosmetic products. This can be analysed by complexometric titration.
- It is used to analyse urine samples.
References
- Vogel`s Textbook of quantitative Inorganic Analysis
- A.H. Beckett and J.B. Stenlake: Practical Pharmaceutical Chemistry, Vol I & II.