A cation is a positively charged species formed by the removal of one or more electrons from its valence shell while an anion is a negatively charged species formed by the addition of one or more electrons in its valence shell. The cations contain fewer electrons than their protons whereas anions contain more electrons than protons.
The word ion is derived from an ancient Greek word ἰόν meaning “going”. In 1834, English chemist Michael Faraday introduced the term ion (suggested by William Whewell), which moves in an aqueous medium from one electrode to the other.
In 1884, Svante Arrhenius published his thesis on “Recherches sur la conductibilité gavanique des électrolytes” (Investigations on the galvanic conductivity of electrolytes). He explained the fact that solid crystalline salt dissociates into its ions. He won the 1903 Nobel prize in chemistry for this explanation.
Prerequisite Concept(s)
- Atomic Structure
- Electronic Configuration
When an atom or group of atoms lose or gain electrons, they convert into ions. In an atom, the number of protons and electrons are equal, that is the reason for its neutrality. The number of protons in an atom almost never changes (unless some radioactive reaction takes place) but the number of electrons can be changed either by gaining or losing electrons. Based on the change in the number of electrons, ions may be positively charged (cation) or negatively charged (anions).
Difference between cation and anion
| Cation | Anion |
| A cation is a positively charged species, formed by the removal of one or more electrons from its valence shell | An anion is a negatively charged species, formed by the addition of one or more electrons into its valence shell |
| Generally, metallic atoms form cations | Non-metal atoms usually form anions |
| Cations are attracted towards the cathode (negative electrode) | Anions are attracted towards the anode (positive electrode) |
| Cations have fewer electrons than protons in the ions | Anions have more electrons than protons in the ions |
| The sizes of cations are smaller than the original atoms | The sizes of anions are larger than the original atoms |
| Carbon cations are termed carbocations | Carbon anions are termed carbanions |
| Cations occupy space between two anions (interstitial space) in the crystal lattice | Anions occupy most of the space in the crystal lattice |
|
Some examples of cations are, K+, NH4+, Ba2+, and Al3+ |
Some examples of anions are SO42-, CO32-, and Br– |
Explanation for cations
Let us take an example of sodium (Na+)
The electronic configuration of sodium is:
11Na = 1s2, 2s2, 2p6, 3s1
By electronic configuration, there are two possibilities for sodium (Na) atom to gain stability. The first one is that it can accept 7 electrons and complete its octet, which is not feasible. The second one is that it can just release one electron from the valence shell (3rd shell) and gain a noble gas configuration. So, it is convenient for sodium (Na) and other similar metals to lose their electrons to become stable.
Electronic configuration of sodium ion is:
(Na)+ = = 1s2, 2s2, 2p6
Explanation for anions
Let us take an example of chlorine (Cl–)
The electronic configuration of chlorine atom (Cl) is:
17Cl = [Ne], 3s2, 3p5
The electronic configuration (E.C) of chlorine (Cl) shows that there are two possibilities for it to get stability. The first one is that chlorine may releases 7 electrons to gain the E.C of noble gases, same as [Ne] and become stable or gain 1 electron to obtain the E.C similar to argon [Ar]. It is obvious for chlorine to gain one electron and form a chloride ion. Other non-metals also tend to gain electrons this way.
Electronic configuration of chloride ion is:
(Cl)– = [Ne], 3s2, 3p6
Additional Resources:
Key Takeaways
Concepts Berg
How can I remember the difference between cation and anion?
Memorize the difference between cation and anion by the following mnemonic:
A cat has paws, which means cation is ‘paw’sitive.
A negative ion sounds N-ION, so, it is easy to remember that an anion is a negative ion.
What are the examples of anions?
The examples of anions are as follows:
Monoatomic anions: Cl–, O2-, N3-, S2-, Br–, F–, I–
Polyatomic anions: CO32-, SO42-, PO43-, NO3–
What do metals form, cations or anions?
Generally, metals form cations. As they have a few numbers of electrons in their valence shell, they tend to lose electrons to obtain stability or a noble gases’ configuration. While sometimes, they also form anions by gaining electrons e.g. natride (Na–), Kalide (K–), Rubidide (Rb–), etc.
Does nitrogen form a cation or anion?
Nitrogen in diatomic form is electrically neutral. But in ionic form, nitrogen can either form an anion such as nitride (N3-), or a cation such as ammonium (NH4+).
Can metals become anions?
Yes! As in the case of alkalides, metals such as sodium (Na), potassium (K), rubedium (Rb), cesium (Cs) can form a negative ion. These species are called Natride (Na–), Kalide(K–), Rubidide (Rb–), and Caeside (Cs–), respectively. These anions are stable in any compatible solvent as well as in a crystalline state.
Examples of cation vs. anion formulae:
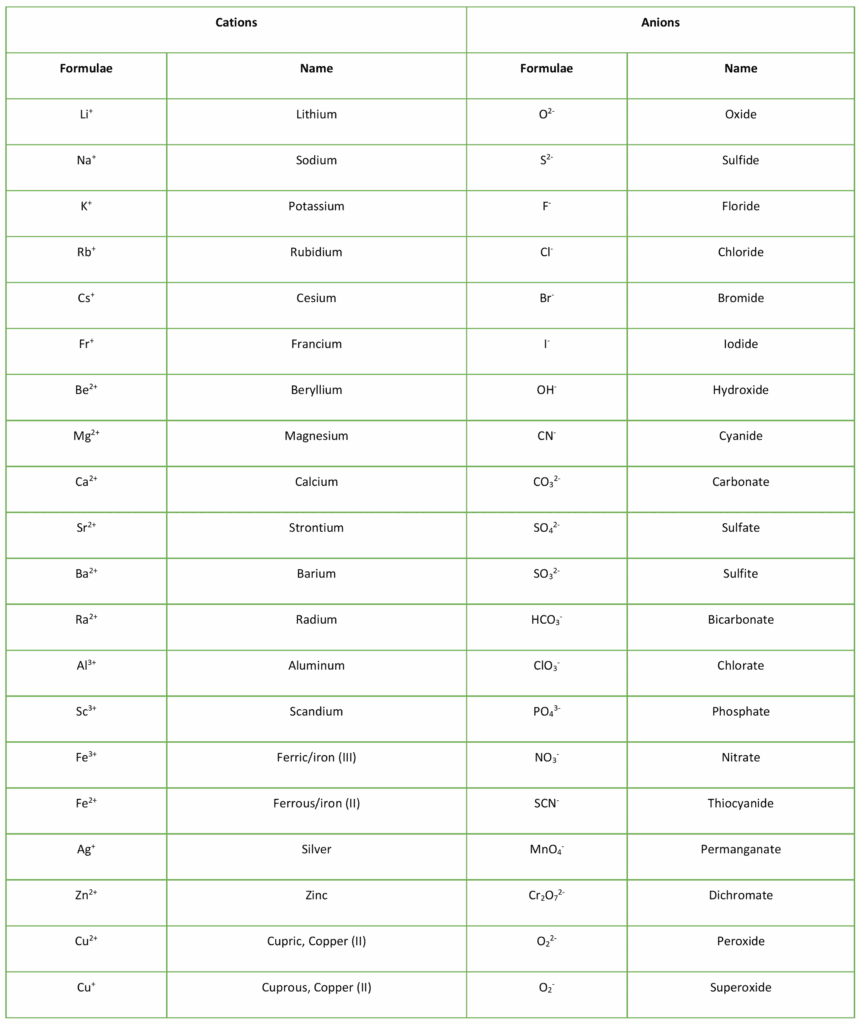 Why is a cation smaller than a neutral atom and an anion larger than a neutral atom?
Why is a cation smaller than a neutral atom and an anion larger than a neutral atom?
The cation and anion of the same neutral element are different in their sizes. In cation, electrons are lost from the valence shell, and there are fewer electrons, so it has a greater nuclear pull, resulting in a smaller size. In case of an anion, electrons are added to valence shell, so there are more electrons in valence shell resulting in decrease in the effective nuclear charge because of the repulsions between adjacent electron pairs. This results in the greater size of an anion than a neutral atom.
What is the polarizability of anions?
Polarizability is the extent to which the electron cloud of a molecule can get distorted. The polarizability of an anion depends upon the size and charge of the molecule.
α = p / E
where
α = Polarizability ( Unit = C m2 V-1 )
p = The ratio of induced dipole moments
E = Strength of electric field that induces polarization
Polarizability comparison of anions
The larger-sized and same charged anion has greater polarizability if the cation is the same.
Comparison of polarizability of halogens as anions is provided:
F– < Cl– < Br– < I–
If the size of anions is the same, the greater charge has more polarizability.
Examples
S2- > Cl–
This means that the lower charge density of an anion increases its polarizability.
What is the difference between ions and charge?
The difference between ion and charge can be summed up as:
Charge
The charge is the property of any matter by which it exhibits attraction or repulsion to any other charged field.
For example; An electron or a proton can be termed a charge.
Ion
Ion is the unequal ratio of protons and electrons in an atom or group of atoms.
For example: Metallic ion (Na)+ , Non-metallic ion (Cl)–
Are anions and cations present in plants?
Cations and anions are present in plants where they perform vital and life-running processes. Functions of some cationic and anionic ions in plants are provided below.
- Magnesium ions (Mg2+) are present in the chlorophyll and help in photosynthesis. The deficiency of Mg2+ ions may cease the photosynthetic process and leaves may turn yellow.
- Calcium ions (Ca2+) are present in plants to make signaling and response mechanisms workout properly.
- Potassium ions (K+) are present in plants to ensure optimal plant growth.
- Nitrate ions (NO3–) are necessary for making amino acids in plants.
- Chlorine ions (Cl–) are present in plants to function for photosynthesis, osmotic adjustments, and suppression of plant diseases.
- Phosphate ion (PO43- ) and Orthophosphate ion (HPO4– ) are involved in key plant functions like photosynthesis, the transformation of sugars, movement of nutrients within plants, and the transfer of genetics from one generation to the next.
References
- Cation and anions (vedantu.com)
- Cation vs. anion (thechemistrynotes.com)
- Alkalide (wikipedia.org)