Chromatography is a group of separation methods. The word chromatography comes from two Greek words; “chroma” meaning ‘color’ and “graphien” meaning ‘writing’. Chromatography was invented by a Russian scientist ‘Michael Semyonowish Tsvet’ in 1903.
Thin layer chromatography, also known as solid-liquid chromatography, is a separation technique in which solid stationary phase and liquid mobile phase are used to separate the components of the analyte on the basis of their relative affinities.
Principle of TLC
The basic principle of thin layer chromatography is the distribution of components based on relative affinities (interactions) with the stationary and mobile phases. The sample component with a higher affinity with mobile phase will move faster while the component with a higher affinity with the stationary phase will move slowly.
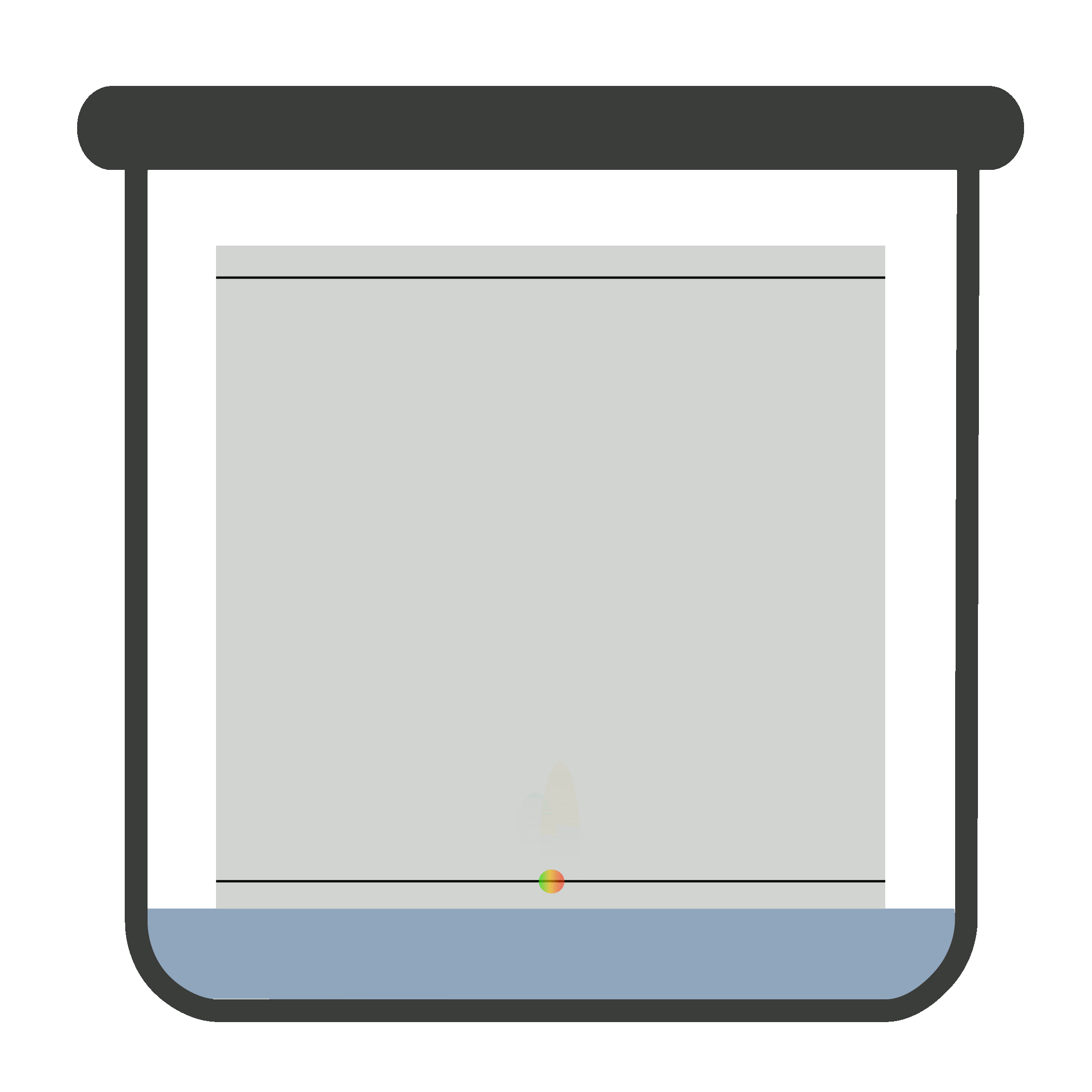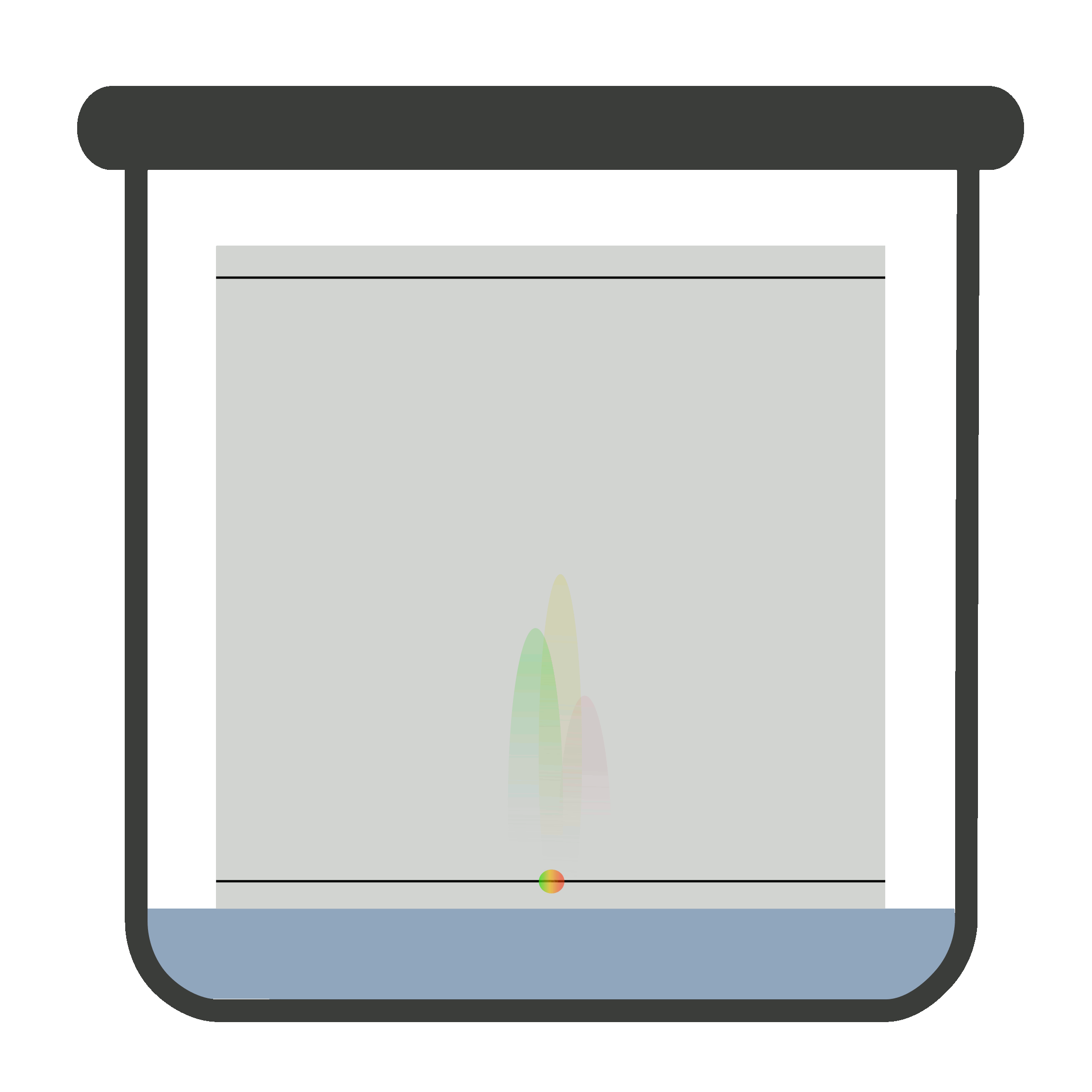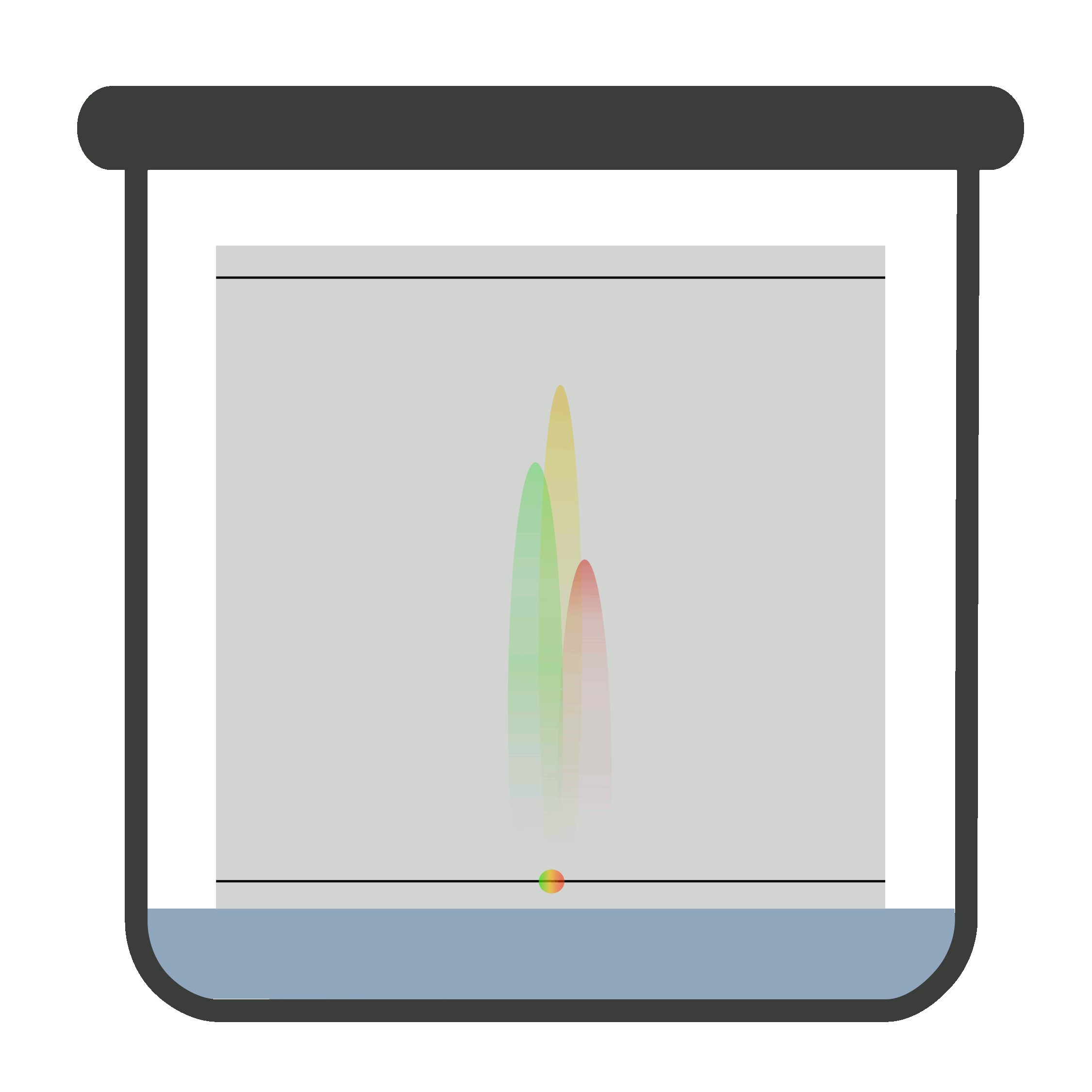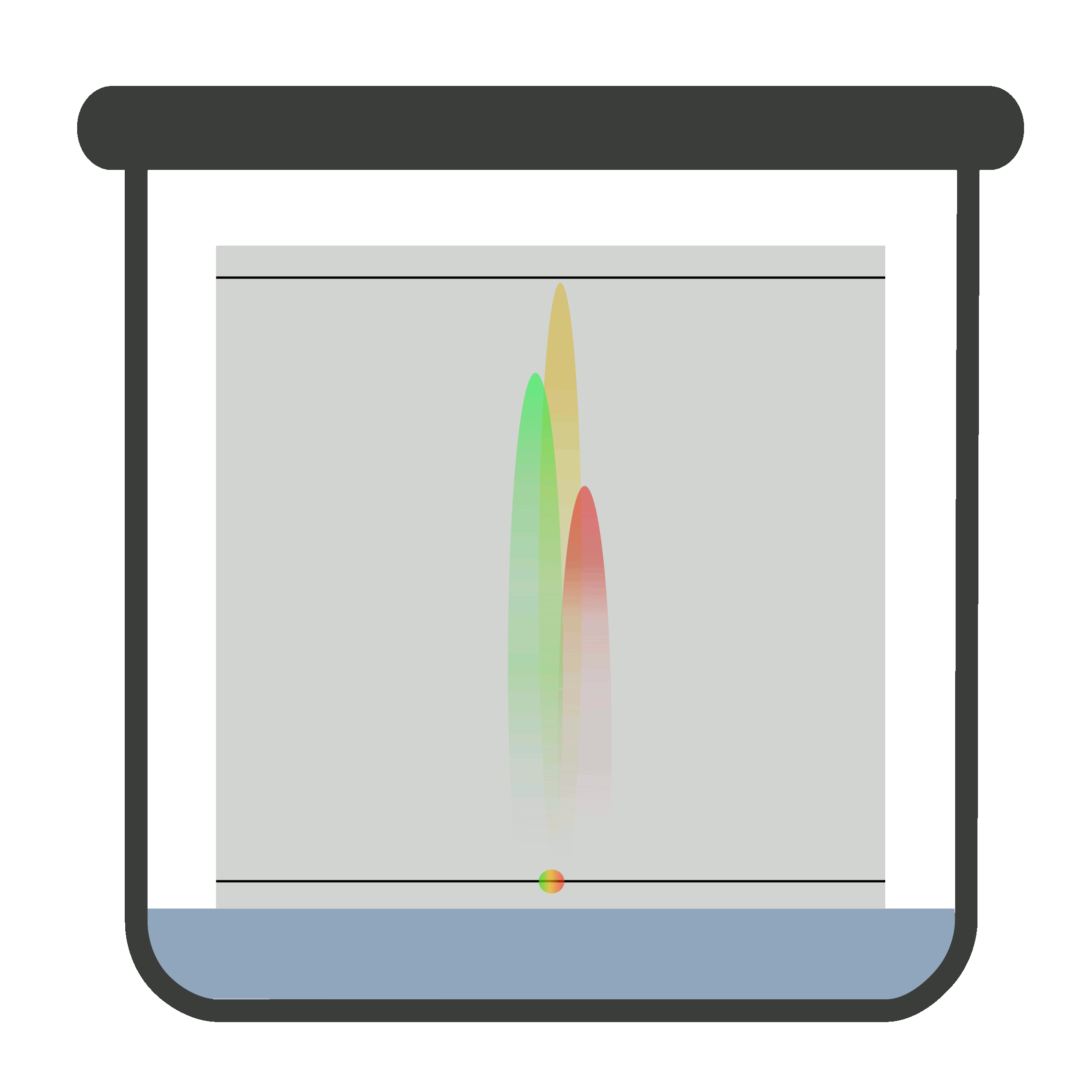
Requirements for TLC
- Suitable solvent
- Suitable adsorbent
- Glass plate
- Appropriate device to apply the thin adsorbent layer
- A suitable device for holding the plate
- Tank or glass jar
- Chromogenic agent or UV lamp
Essential steps in TLC
- Choice of stationary phase
- Choice of mobile phase
- Preparation of TLC plate
- Sample application
- Development of chromatogram
- Quantification of separated components
1. Choice of stationary phase
Mostly the adsorbent used in thin layer chromatography is a thin layer of silica gel or powdered cellulose on inert support of glass plate, plastic, or aluminum foil. The commonly used adsorbents are as follows:
For weak polar compounds
- Activated charcoal
- Alumina
For polar compounds, amino acids, and sugars
- Silica gel
- Cellulose
- Polymeric resins (styrene, divinylbenzene)
2. Choice of mobile phase
The mobile phase should be a volatile liquid as solvent. Generally, benzene, ether, and ethanol are used in TLC. The choice of mobile phase depends upon two factors:
- The nature of substance to be separated
- Nature of the adsorbent
In case of polar analytes, when the stationary phase is silica or cellulose, the mobile phase must be polar. Whereas for nonpolar analytes where alumina or activated charcoal are used as a stationary phase, the mobile phase must be a non-aqueous solvent. Note that the combination of solvents as a mobile phase gives better separation.
3. Preparation of TLC plate
An aqueous slurry of silica gel or powdered cellulose (stationary phase) is spread uniformly over a clean surface of a metal, glass, or plastic plate, or micro slide with the help of a spreader.
A binder may also be added to the slurry to enhance the adhesion of the adsorbent. The recommended layer thickness is 150 – 250µm. Leave the plate to air dry overnight or oven dry at 100 -110℃ for 30 minutes. This is called activation of TLC plate. Removal of residual water is necessary for the activation of TLC plate. If the plate is not activated, the residual water may act as a stationary phase. The plate gets ready for use once activated.
Ready to use plates are also available commercially. They are conventional and offer high performance with 200 -250µm thickness with 20µm or greater particle size. Commonly available dimensions of conventional plates are
5×20, 10×20, 20×20 (cm x cm)
Here are some precautionary measures that should be followed during the preparation and activation of the TLC plate:
- Care should be taken in handling to avoid placing fingers on the active adsorbent surface. And to avoid extraneous substances on it
- Pre-washing of the plate is recommended to wash out the extraneous substances within the plate. Washing of plate is done by running the developing solvent on the plate
- Water should completely be removed from the slurry
4. Application of analyte
The sample is usually applied at least 1 – 2cm distant from the bottom of the plate. The sample spot should be applied precisely to make quantitative analysis easy. Make the spot compact, 2 – 3mm in diameter.
A syringe or capillary tube can be helpful in the sample application. Mechanical dispensers are also commercially available.
Let the spot dry before proceeding.
5. Development of chromatogram
Place the plate in the closed chromatographic container. Be sure the container is saturated with vapors of the developing solvent or mobile phase. One end of the plate is immersed in developing solvent.
The sample must not be in contact with the developing solvent. When the mobile phase travels to the solvent front, remove the plate carefully and let it dry. Now, locate and mark it.
It is compulsory to remove the TLC plate from the chromatographic container when mobile phase reaches solvent front because if it is not removed timely, the solute (sample) may elute with the solvent.
6. Measurement and Identification of solutes
The spots may be located by various methods after separation.
Visible or colorful spots can be seen with the naked eye. Colorless species may be seen by using physical or chemical methods of visualization. Here are some methods for visualizing the spots.
Spraying a chromogenic agent
The chromogenic agent is a compound that gives color in reaction. Usually, iodine vapors or sulphuric acid spray is used as a spraying agent. These two compounds, when reacting with organic compounds, form dark products. Iodine vapours give dark brown spots after the reaction. Sulphuric acid spray also gives dark spots when reacting with organic compounds. Ninhydrin is used to locate amino acids.
Here are some other chromogenic agents that are being used
- Aniline pthalate is used to detect sugars.
- Antimony trichloride is used in case of cardiac glycosides.
- Potassium chromate is used to visualize lead. It gives yellow spots with lead (II) and orange with mercury (II).
- Rubeanic acid is also used as a chromogenic agent. It gives a blue spot with nickel (II), brown with cobalt (II), olive green with copper (II), and brownish-green with iron (II).
Exposure to Ultraviolet (UV) rays
Another method for visualization of spots is to incorporate a fluorescent material into the stationary phase and then examine it under ultraviolet light. It is a non-destructive visualization method. The solute can be visualized as a dark spot in front of a fluorescent background under ultraviolet light.
Radiometry
Radiometry is also used for the visualization of spots in thin layer chromatography.
Two-dimensional chromatography (2D TLC)
Sometimes, the results overlap causing a difficult identification and calculation. The reason being inability of a single solvent to separate the components of the analyte completely. Thus, a 2D TLC is used in which two mobile phases separate analyte against a stationary phase in two steps.
A square TLC plate is required for two-dimensional chromatography. The second run is done perpendicular to the first one.
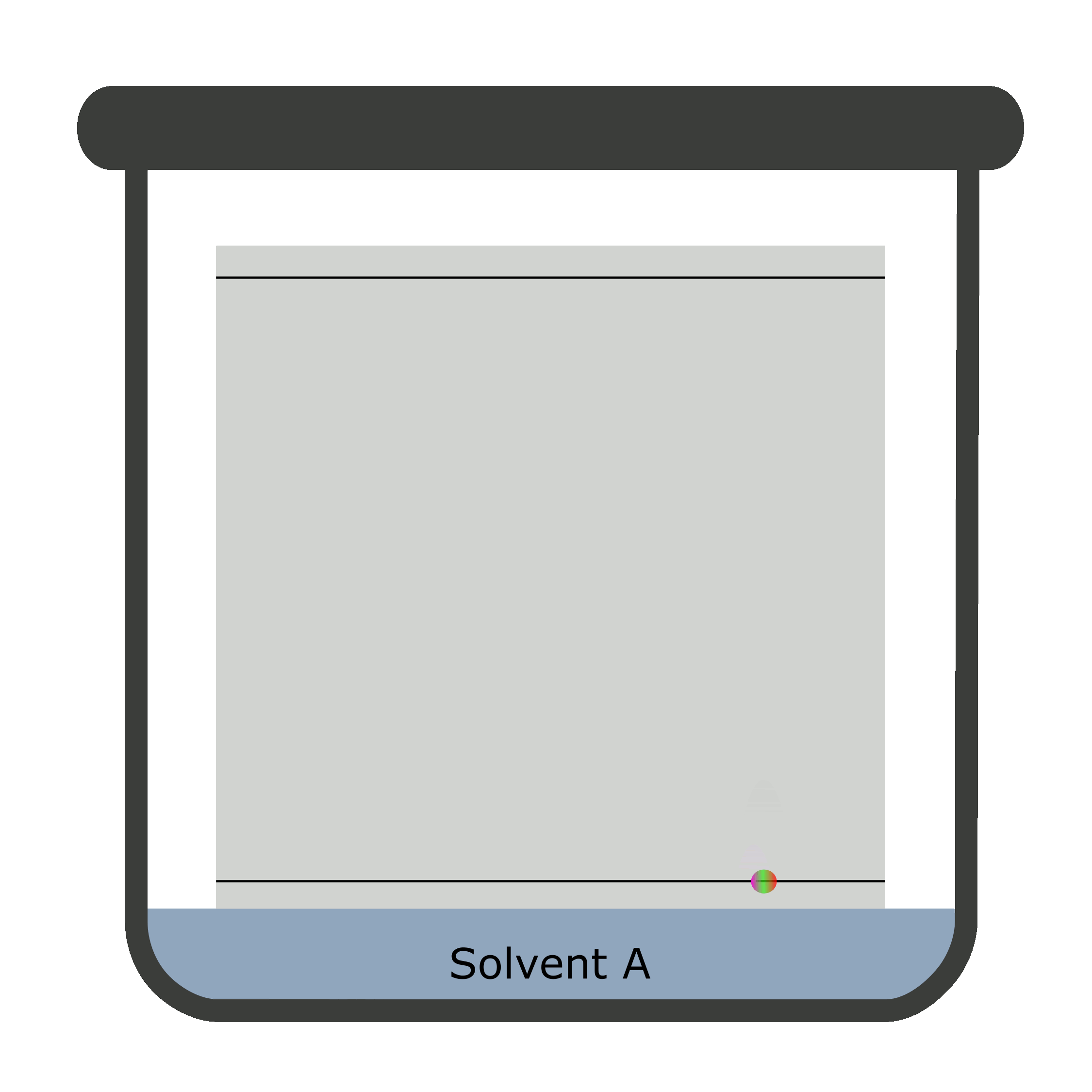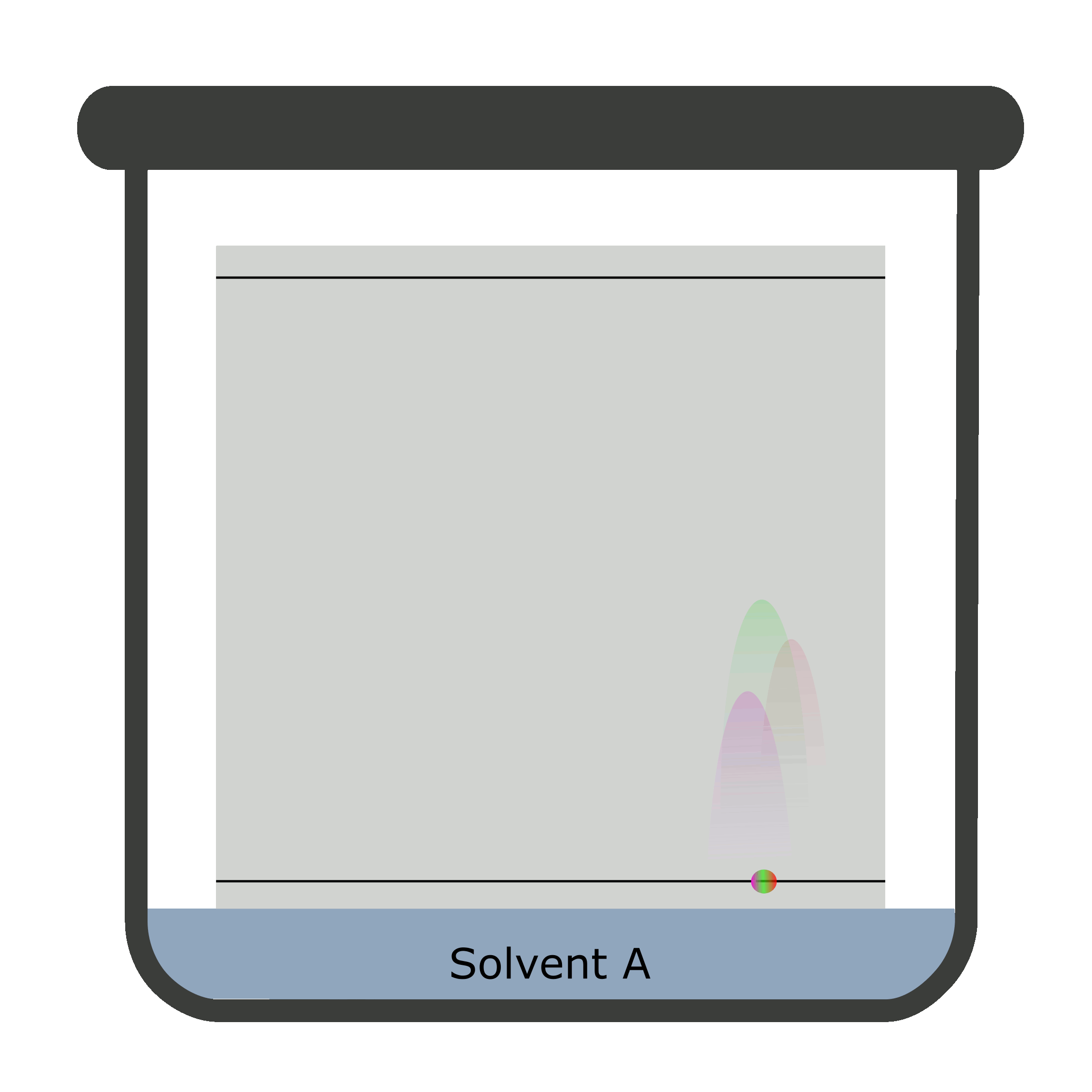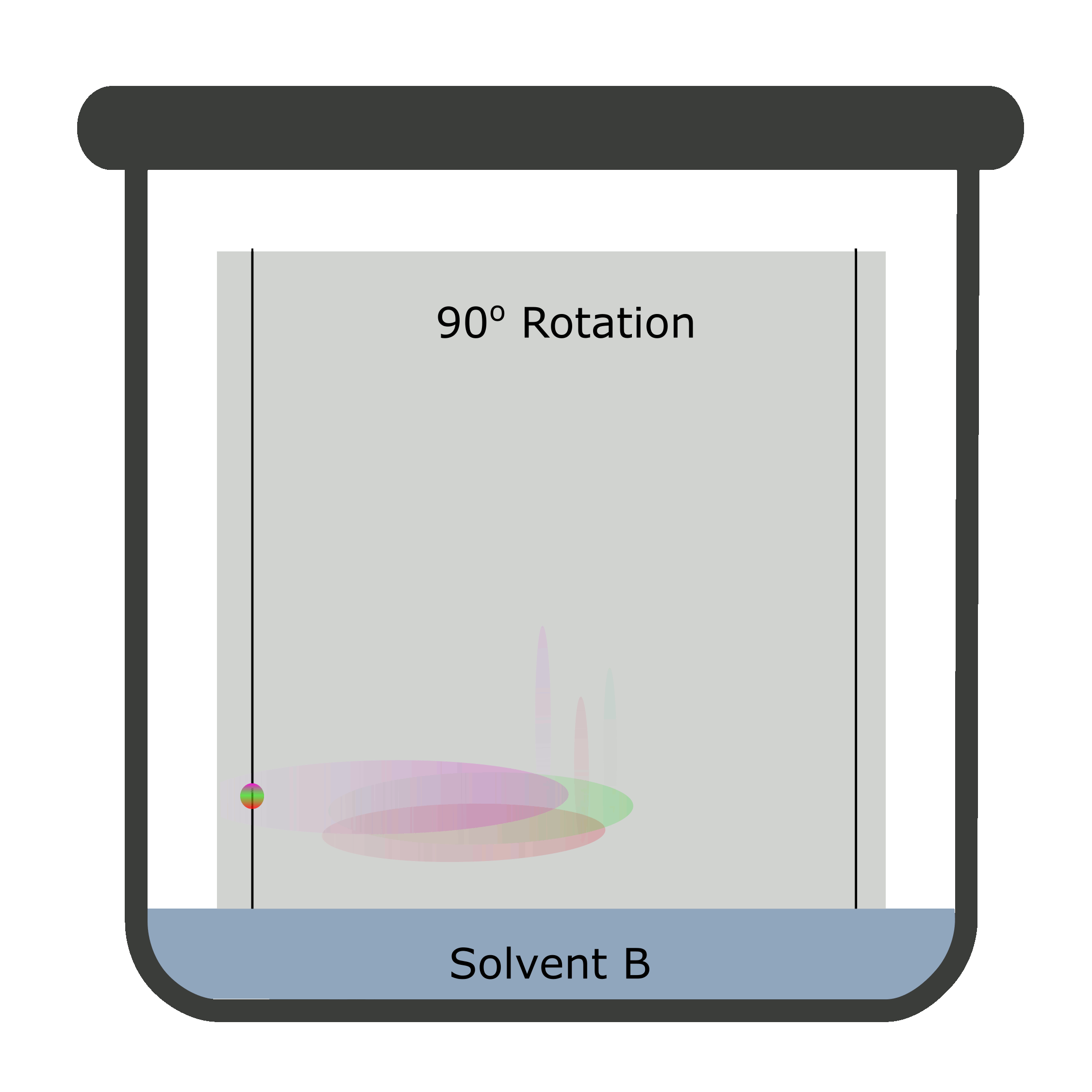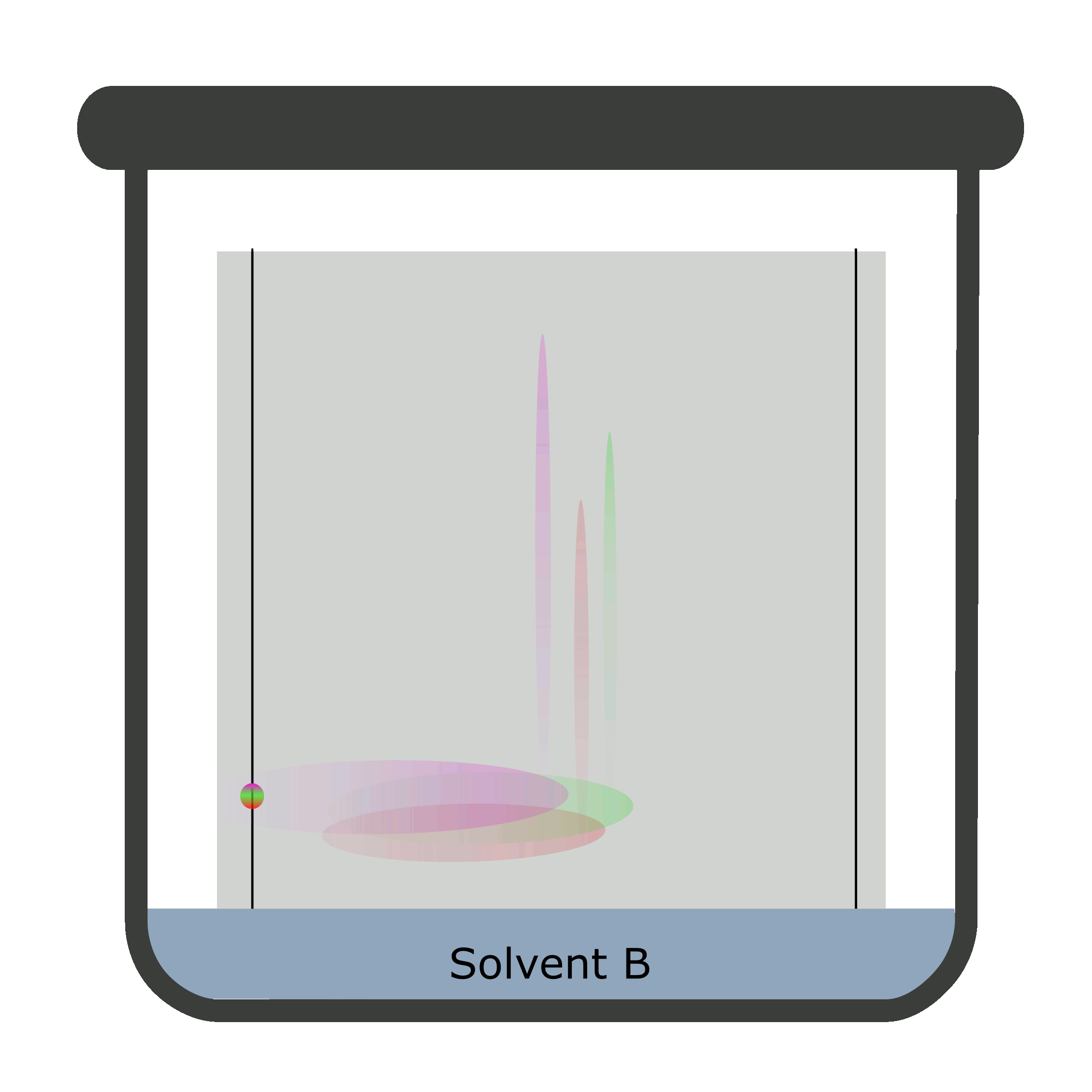
Calculation of Rf values in TLC
Rf stands for ‘retardation factor’ or ‘retention factor’. It is taken as the ratio of the distance traveled by the components of a mixture to the distance traveled by a solvent. It is expressed as:
Rf = distance traveled by the solute / distance traveled by the solvent
The less polar component of the mixture that has more affinity with the non-polar mobile phase will elute faster getting the highest Rf value and the more polar component of the mixture having more affinity with the polar stationary phase will elute later with the least Rf value and vice versa.
Rf value is also dependent on some other factors. Under identical conditions these factors are:
- The particle size of the adsorbent
- The thickness of the adsorbent
- Composition of the solvent and its quality
- The degree of saturation of the tank
- Prior activation and storage conditions of the TLC plate
Quantitative evaluation
It is a semi-quantitative estimation of components of a mixture by scraping the spot from the plate. The analyte is then extracted from the scrapped solid. The analyte concentration is measured using a physical or chemical method.
In the end, the radiation emitted from the spot by fluorescence is measured by a scanning densitometer.
Applications of TLC
Thin layer chromatography is used for the identification, purification, and isolation of components of a mixture. In addition to this:
- Thin layer chromatography has various applications in clinical and forensic areas.
- It is used for the separation and recovery of dyes.
- The separation of carbohydrates is also done with TLC.
- Another use of TLC is the separation of artificial colorants in sugar confectionery.
- Being used in purification processes, it helps to keep in check the progress of purification by molecular distillation and to check the distillation fractions.
- It is also used in the identification of plant extraction, drugs, and adulteration in food products.
- TLC is used to study organic reactions i.e. in reaction kinetics.
- Characterization and isolation of alcohols, acids, amino acids, vitamins, amides, glycols, and alkaloids can be done with the help of thin layer chromatography.
- It is used for the identification of products in many reactions.
- It is also used to check the purity of products in organic synthesis.
- Another use of TLC is in the separation of metals like nickel, copper, zinc, and manganese in qualitative analysis.
Advantages of TLC over paper chromatography
- TLC is a time saving technique as compared to paper chromatography.
- TLC is more sensitive and it gives sharp compact spots.
- It is a non-destructive technique as a TLC plate can be dried at a higher temperature.
Disadvantages of TLC
- Thin layer chromatography is applicable only for non-volatile compounds.
- It is a classical technique and does not work as an automated system.
- It has limited resolution capabilities, that is the separation of a large number of components is not possible simultaneously.
- TLC is limited only to semi-quantitative analysis.
Comparison of TLC with CC and HPLC
- HPLC is far faster, more reliable for quantitative analysis, and gives greater resolution than TLC and CC.
- TLC can run more samples simultaneously when compared to CC and HPLC.
- TLC is cheap as compared to other chromatographic techniques.
- It is a versatile technique i.e. TLC can detect almost every type of chemical compound.
Concepts berg
What Is Thin Layer Chromatography?
In simple words, TLC is a separation technique. It is used to separate non-volatile mixtures. The separation is based on the relative affinities of sample components with the stationary and mobile phases.
Who discovered thin layer chromatography?
Thin layer chromatography was first used by two Russian scientists N.A. Izmailov and M.S. Schreiber in 1938 for separation of plant extracts. They used a slurry medium of 2mm thickness. The plant extract was applied at the center of the layer. The separation was in ring-like shapes. This work was further reviewed in 1941 by M.O’L Crowe. The first reported TLC in analytical chemistry was improved by adding binders in sorbents by J.E. Meinhard and N.F. Hall in 1949.
What are the two phases of thin layer chromatography?
TLC has two phases. A thin layer of silica or alumina on inert support acting as a solid stationary phase. The second one is a developing solvent that acts as a mobile phase.
Describe thin layer chromatography’s solvent selection?
When separating unknown mixtures, analyze solvents one by one, starting with less polar. A mixture of a polar and a non-polar solvent can be used.
Commonly used solvents are acetone (CH3COCH3), n-hexane (C6H14), acetic acid (CH3COOH), methyl alcohol (CH3OH), benzene (C6H6), and chloroform (CHCl3).
Is thin layer chromatography an adsorption chromatography?
Adsorption chromatography is a type of chromatography that involves surface adsorption. And TLC is categorized as adsorption chromatography as it uses a thin layer of adsorbent (silica).
What does the TLC Rf value mean?
Rf stands for retention factor. This value indicates the affinity of different solutes with the stationary and mobile phases. It is the ratio between the distances of the sample component (the solute) and the developing solvent. It ranges from 0 to1. Rf value equal to zero indicates no (or less) affinity with mobile phase. While the Rf value 1 indicates more affinity with the mobile phase.
The greater the distance traveled by the sample component, the greater will be its Rf value. Having more affinity towards the mobile phase, it would elute first.
When the distance traveled by any component is less, this means greater affinity with stationary phase thus, elution would be late.
Why is silica gel used in TLC?
When we intend to remove the water content or moisture from the TLC plate, silica gel is heated at 100 -110°C for activation. Silica gel is preferred as it is stable at high temperatures and does not interact with any mobile phase.
What does TLC say about purity?
A pure compound gives only one spot on the plate. More than one spot indicates impurities and so do gradients.
Is silica polar or nonpolar?
Silica (SiO2) is a fine, pure sand powder in which silicon and oxygen atoms are arranged in a definite space. It is highly polar because of the bond polarity of Si-O.
What are commonly used stationary phases in TLC?
The commonly used stationary phases are:
- silica gel
- alumina
- activated charcoal
- powdered cellulose
- polymeric resins
Why should the start line stay above the solvent in chromatography?
The baseline must lie above the solvent. This avoids contamination of solvent with the analyte. Moreover, it is needed for proper elution, separating all the analyte components as per their affinity to the stationary and mobile phases.
Which one is better in TLC, the higher or the lower Rf value?
The best Rf value in thin layer chromatography ranges from 0.3 to 0.7. The values in this range make the understanding, evaluation and comparative analysis easier. If the separation of any component is not complete or proper, it can be confirmed with two dimensional thin layer chromatography.
Why do we use UV light to detect the organic compounds on a TLC plate?
Organic compounds contain chromophores. Change in absorption shift occurs when the chromophores are exposed to UV light. Hence, the spots become visible under the UV lamp.
How are amino acids separated by TLC?
Amino acids form bright colored compounds with ninhydrin (chromogenic or locating agent). So, they can be easily differentiated from other compounds.
Why do we use iodine crystals in thin layer chromatography? How does it help?
When exposed to iodine vapors, the spots on a developed TLC plate become visible.
What is the role of HPTLC and TLC in food analysis?
Thin layer chromatography and HPTLC have a great role in the analysis of food components in the food industry. Many food components are separated with the help of thin-layer chromatography, including:
- Separation and quantification of amino acids in milk, orange juice, and fruits.
- Determination of aromatic alcohols, sorbitol, and glucose in foods.
- Identification and quantification of carboxylic acids and ethanol in meat and various foodstuffs.
What is the major difference between thin layer chromatography and paper chromatography?
The difference between paper chromatography and thin layer chromatography is the type of stationary phase used. In TLC, the stationary phase is a thin layer of solid adsorbent on an inert support. The adsorbent often used is silica gel. While in paper chromatography, the water content present in cellulose or paper will act as a stationary phase.
Paper chromatography is liquid-liquid chromatography while thin layer chromatography is solid-liquid chromatography.
How is thin layer chromatography more superior to other chromatography methods?
Thin layer chromatography is a simple, fast, cheap, portable, versatile technique and it is not a laborious one. That’s why it is, sometimes, called a superior technique to other chromatographic techniques.
Are thin layer chromatography and paper chromatography stationary phases the same?
The stationary phase in thin layer chromatography is silica (solid) while in paper chromatography, the water molecules (liquid) present in the cellulose of the paper acts as the stationary phase.
Why would one use TLC over paper chromatography?
TLC is preferred over paper chromatography as it is time saving, more sensitive, non-destructive, and temperature resistant technique. Furthermore, TLC is more precise and accurate than paper chromatography.
Why is TLC used in organic chemistry?
TLC is used in organic chemistry due to its vast applications in the field. It is useful in knowing the purity of compounds, the reaction kinetics of many organic reactions, identification of products, and the characterization of various organic compounds.
How does oil from your fingers affect the thin layer chromatography?
Oil from fingers may interact with silica or with the resulting spots thus, affecting the results.
Should the stationary phase be necessarily thin in thin layer chromatography?
If the layer is thick, there is a chance of absorbance of the analyte in the thick layer instead of adsorption.
Why are volatile solvents used in thin layer chromatography?
Solutes are invisible in the presence of a solvent as the solute particles get diffused with solvent. The volatile solvent must be used so that the evaporation and drying of the plate become easy.
How often is thin layer chromatography conducted in the monitoring of a reaction?
To monitor a reaction’s progress, one can take the reaction mixture after several intervals and run on TLC. As pure compounds give only one spot, it can indicate the completion of the reaction.
Can I separate solid components using thin layer chromatography?
The solid components cannot be separated by thin layer chromatography. The technique is only suitable for the samples that are in the same phase as that of the mobile phase i.e. liquid.
Is it possible to perform thin layer chromatography to separate micelles from a solution?
TLC is a surface adsorption phenomenon thus, it cannot be used for the separation of micelles from a solution.
References
- Vogel textbook of Quantitative and Qualitative analysis
- Fundamentals of analytical chemistry by Skoog and West 9th edition
- Analytical techniques and instrumental analysis by Mahinder Singh