There are many biochemical processes taking place in living organisms that are pH sensitive i.e. They are usually enzyme catalyzed reactions and if the pH of these processes is altered, they cease to occur. That is where buffers come into play.
Definition of a buffer
Buffer is a chemical substance that maintains the pH of a biological system when a small amount of acid or a base is added to it.
In living organisms, buffers are important because they resist sudden changes in the pH of body fluids of living organisms:
- Bicarbonate buffer maintains the pH of the blood.
- Phosphate buffer maintains the internal environment of cells.
- Hemoglobin has buffering capacity.
- Proteins have a zwitterionic structure that enables them to resist pH change.
Acidic buffers are made up of a weak acid and its salt with a strong base. For example, ethanoic acid and sodium ethanoate solution buffer has a pH of 4.
Principle of Buffer action
When a small quantity of an acid or base is added, the buffer behaves in such a way, to minimize the effect of that acid or base as much as possible so that the pH remains constant. This is known as Le Chatelier’s principle.
Bicarbonate buffer
Bicarbonate buffer is present in human blood. It works to maintain the pH within the normal range (7.35 to 7.45).
The below equations show how it reacts to the addition of acid and base.
Addition of Acid (H+)
HCO3– + H+ ⇌ H2CO3
When a small amount of H+ is added, the equilibrium shifts forward and carbonic acid is formed. The concentration of H+ remains constant.
Addition Of Base (OH–)
H2CO3 + OH– ⇌ HCO3– + H2O
When a small quantity of base (OH–) is added, the carbonic acid returns to its ionic form. Water, a neutral substance, is formed during this process, thus, the pH remains the same.
Phosphate Buffer
Phosphate buffer is present in cells of living things. It maintains the pH of the cell within a normal range. It consists of phosphoric acid (H3PO4) in equilibrium with dihydrogen phosphate (H2PO4–), hydrogen phosphate (HPO42-), Phosphate (PO43-), and hydrogen ions (H+).
The below equations show the reaction of this buffer when acid or base are added.
Step (I) H3PO4 ⇌ H+ + H2PO4–
Step (II) H2PO4– ⇌ H+ + HPO42-
Step (III) HPO4– ⇌ H+ + PO43-
Phosphoric acid is in intramolecular equilibrium. It is present in all of these ionic forms.
Addition of H+
When the addition of an acid (H+) occurs, step (iii) of the reaction goes backward. On further addition, step (ii) goes backward, and if more acid is added, step (i) goes backward too all the way back to reactant (Phosphoric acid). The number of H+ remains constant. This way, phosphate buffer resists change in pH.
Addition of OH–
When OH– is added, it reacts with H+ ions to form water. Water, being a neutral species, does not alter the pH. The buffer is in ionic equilibria. So, hydrogen ions which are consumed to form water, get compensated by the further dissociation of phosphoric acid (Forward reactions).
Hemoglobin
Hemoglobin carries oxygen by binding it up with itself, from the lungs to the muscles through the bloodstream. It can also act as a buffer. The muscles produce hydrogen ions (H+) and carbon dioxide (CO2) during the metabolism process. Hemoglobin tends to bind reversibly with hydrogen ions too, other than oxygen (O2). When one of these substances is bound with hemoglobin, the other one detaches simultaneously.
These hydrogen ions being released by muscles are hereby bound with hemoglobin i.e. pH remains constant. Moreover, the released oxygen is utilized by the muscles.
Protein buffer
Amino acids are the building blocks of proteins. Amino acids contain amide (-NH2) and carboxyl (-COOH) functional groups. They can act as biological buffers because they exist in the form of zwitterions as shown in the figure below.
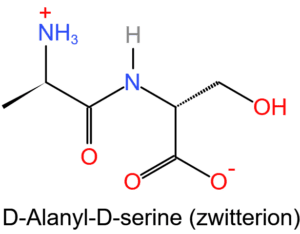
They are formed by intramolecular proton transfer. When hydrogen ion (H+) is added to the system which contains zwitterions, it is immediately captured by carboxylate group (-COO– ) to form carboxylic acid and whenever hydroxide ion (OH–) is added, it will encounter amine group ( -NH3+), i.e. they will react to form H2O (neutral substance) and an amide (-NH2).
Key Takeaways
Buffers are important to living things as many biochemical processes occur within a narrow range of pH, including metabolism, respiration, nerve impulse transmission, digestion, and muscle relaxation.
- Important buffers in living systems (sciencing.com)
- Buffer (chemistry.wustl.edu)