The acidity of an acid can be determined by the stability of its conjugate base. The greater the stability of the conjugate base, the stronger is the corresponding acid. The conjugate base of HF i.e. Fluoride ion (F–) is very unstable because of its very high electronegativity. Fluoride (F–) attacks back on hydronium ion (H3O+) as soon as it forms, hence, reducing the hydrogen ion (H+) donation ability of the acid.
Due to this reduced acidic property, H+ ions are only available in minute quantities in the solution. That is the reason, why hydrofluoric acid (HF) is a weak acid i.e. It does not ionize completely when added to water.
Another reason for the weakness of HF as acid is the presence of hydrogen bonding, but only if ‘HF’ is either pure or added to protic (H+ containing) solvent having (F, O, or N) attached directly to the H atom. In this case, the negative part of HF i.e. F– is strongly attracted to the proton of the solvent. i.e. reducing the acidity of HF.
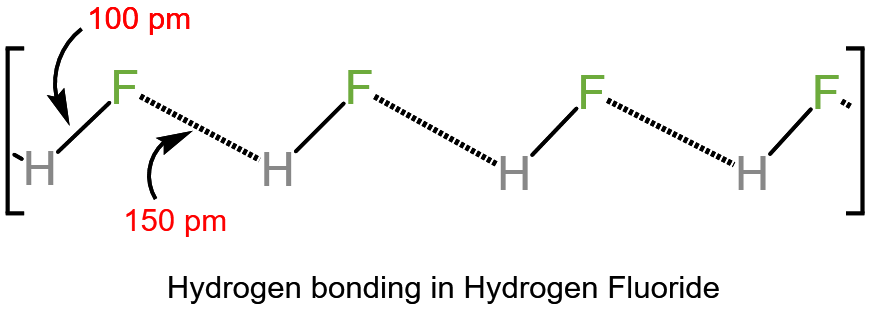
This property makes HF, a very bad reducing agent. Thus, it can be used in such places where reduction proofing is required.
Related Links